ILA: Our Biotech Pick of the Year
Disclosure: S3 Consortium Pty Ltd (the Company) and Associated Entities own 3,265,720 ILA Shares at the time of publishing this article. The Company has been engaged by ILA to share our commentary on the progress of our Investment in ILA over time. This information is general in nature about a speculative investment and does not constitute personal advice. It does not consider your objectives, financial situation, or needs.
Our 2025 Biotech Pick of the Year is Island Pharmaceuticals (ASX:ILA).
The US Federal Drug Agency (the FDA) has just agreed that ILA’s drug for the Marburg virus is eligible for fast track approvals and a valuable Priority Review Voucher.
ILA has effectively been approved to skip the 15+ year, expensive, process of human phase 2 & 3 clinical trials.
With this now in hand, we have named ILA our 2025 Biotech Pick of the Year.
ILA’s Galidesivir drug has the potential to defend against bioweapons.
“Biological warfare” is the deliberate use of biological agents like bacteria, viruses, or other toxins to harm or incapacitate humans during conflict.
ILA’s drug has already been shown to be effective against ~20 viruses in lab tests - many of which sit in the “weaponisable” category.
We had been waiting for months to find out if the US FDA would advise whether or not ILA’s Galidesivir drug for the Marburg virus is eligible for “fast track approvals” and a valuable “Priority Review Voucher”...
This morning ILA announced that the FDA said yes:

(source)
ILA has announced a zoom webinar where management will talk through the FDA’s response in detail:
(Register for ILA’s webinar today - Monday 17th November 10:30am AEDT)
The FDA uses its “Animal Rule” to fast track approval of drugs when traditional human trials are not ethical or feasible—such as treatments for diseases caused by dangerous chemical, biological, radiological, or nuclear agents.
ILA now has a path to FDA approvals and commercialisation of its Galidesivir drug for Marburg virus within 12 months...
... which could mean US government stockpiling contracts soon after.
Next, we need to see ILA confirm a trial design with the FDA, run new animal trials and then submit the data for approvals with the FDA.
Another big unlock of accelerated approvals for ILA is eligibility for a “Priority Review Voucher” (PRV).
A Priority Review Voucher is a tradable certificate that lets a drug company shorten the US FDA’s review time for a future drug application - as a reward for having developed a treatment for certain diseases.
These “tradable” PRVs are inherently valuable on their own because companies can sell them to other companies... on average, they are selling for US$100M (A$156M).
ILA is currently capped at $108M.
ILA finalised its acquisition of its Marburg drug earlier this year. Ever since then, the main catalyst we wanted to see for the asset was a decision on Animal Rule fast track eligibility by the FDA.
Which is why with eligibility now confirmed by the FDA, we have named ILA our 2025 Biotech Pick of the Year.
Basically ILA now owns a Phase 2/3 stage asset for Marburg virus...
(if the FDA had said no, it would have taken ILA years to get to this stage using human trials)
Marburg virus is classified as a Category A bioterrorism threat (the highest level threat) by the US government (source).
Marburg is the only Category A biothreat with no current vaccine or FDA approved treatment...
It's also one of the most deadly with a fatality rate up to 88%.
ILA’s animal trials for Marburg have shown survival rates of up to 94% versus 0% survival in placebo.
Like Ebola, Marburg outbreaks can occur naturally too (but thankfully are very rare and usually elicit a rapid coordinated response to try and contain them due to their severity and high fatality rates).
Over the weekend, the World Health Organisation reported a confirmed outbreak of Marburg in Ethiopia - the first in the country's history:

(Source)(Source)(Source)(Source)
In natural outbreaks, Marburg virus typically spreads to humans from direct contact with infected fruit bats or bat excretions, or through exposure to fluids from infected people during close contact.
Weaponised Marburg virus is whole different story...
Marburg virus can be weaponized by concentrating, stabilizing, and aerosolising the virus in a laboratory to create infective particles intended for dispersal, potentially through bombs, aerosols, or other delivery systems - allowing for airborne transmission, rapid spread, and targeting of populations.
During the Cold War, the Soviet Union's bioweapons program actively weaponised Marburg virus, producing it in large quantities and favoring it over Ebola due to its stability in weaponised form (source, source ).
Urgency to find a cure/treatment is now front of mind.
Which may also mean government stockpiling is now up for consideration a lot more too...
So ILA’s animal trials for Marburg have shown survival rates of up to 94% versus 0% survival in placebo.
Animal Rule eligibility now means ILA can try to get a drug approved fast with new animal trials that are designed based on FDA feedback...
And then if approvals come in, ILA can try turn its Marburg treatment into what management think can be a $200M - $500M cash generator through: (source)
- A Priority Review Voucher (PRV) that comes in if ILA’s Galidesivir is FDA approved - these can be worth on average US$150M.
- US Government national stockpiling deals - these can be worth anything between US$100M to US$1.2BN ANNUALLY (check out that slide below).
ILA mentioned that they have already started on US government contract engagement.

(Source)
ILA’s Marburg drug (Galidesivir) has already had US$70M spent on development (most of which was funded by the US government).
Marburg disease is classified as a Category A bioterrorism threat with a fatality rate of up to 88% in humans - Category A is the highest level threat by the US government. (source)
(for Category A bioterrorism threats, governments around the world will generally maintain a stockpile of vaccines or treatments to quickly deploy).
There is no current vaccine or cure for Marburg (and no strategic stockpile).
Marburg is the only Category A biothreat that has no cure or stockpile right now.
Which explains why the US would have spent US$70M on Galidesivir...
AND why ILA is now “commencing US government engagement initiatives”.
The drug has been tested across ~20 viruses in clinical trials before and is proven to be safe in humans already.
All of that data and work matters because it forms the basis for an application for approvals using the FDA’s “Animal Rule” process”.
The Animal Rule allows for drugs to be fast-tracked to market because of how deadly these conditions can be (and the urgency around defending against bioterrorism and bioweapon threats).
ILA’s drug has shown 100% survival rates for animals 24-48 hours after infection, relative to placebo where survival rates are 0%.
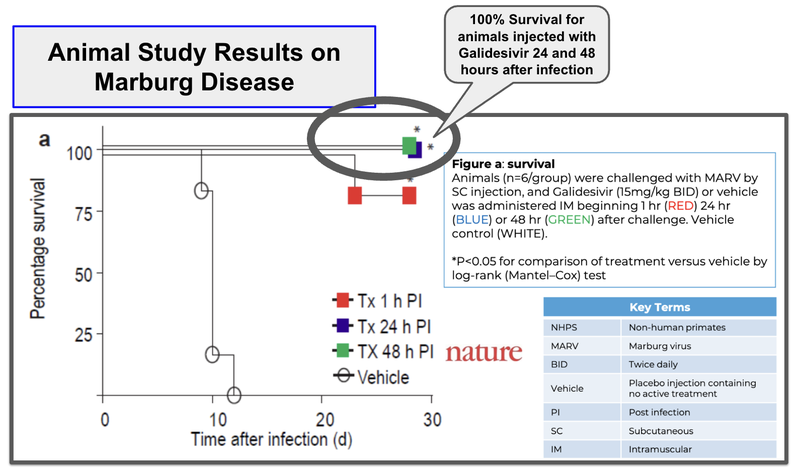
(Source)
Now that ILA has been given the green light to pursue approvals via the Animal Rule:
- ILA will consult the FDA on trial design to address everything the FDA wants to see for final approvals. ILA expects those trials to cost ~$4M) (source). (ILA had $6.9M cash at 30 Sept and ~$1M in the money options) (source)
- UPDATE on the Animal Trials from today: ILA confirmed it was “working with Biosecurity Level 4 (BSL4) facilities where animal studies may be conducted” so that they are ready to go once trial designs are locked in with the FDA.
- THEN, ILA will submit to the FDA for an Investigational New Drug (IND) approval of its drug (6 month review timeframe)
- IF APPROVED...
- SECURE a Priority Review Voucher, which is a tradeable asset worth on average ~US$100M (think of this like a ‘thank you’ for developing the drug)
- SECURE commercial stockpiling contracts with the US Department of Defence for protection against bioweapons. These contracts can be very lucrative.
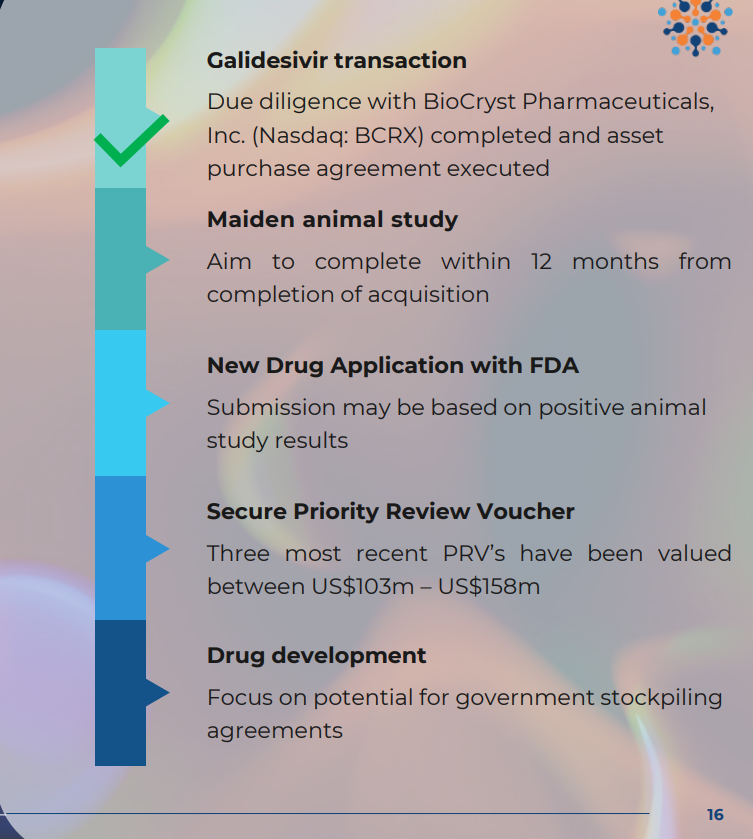
(Source)
In the short term, though, the major catalyst will be locking in a final trial design which ILA could finalise before the 2nd of December which is the due date for clarifying questions to the FDA from ILA.
We are backing the ILA team here...
A big part of the reason why we are Invested in ILA is because of the team behind the company.
Two of ILA’s biggest shareholders (Garner and Tillett) and one of ILA’s directors (Ntoumenopoulos) were part of the Race Oncology team that took that company’s share price from ~6.6c to $4 per share.
Race Oncology, like ILA, was also a drug repurposing company...
We are backing this team to deliver similar success for ILA.
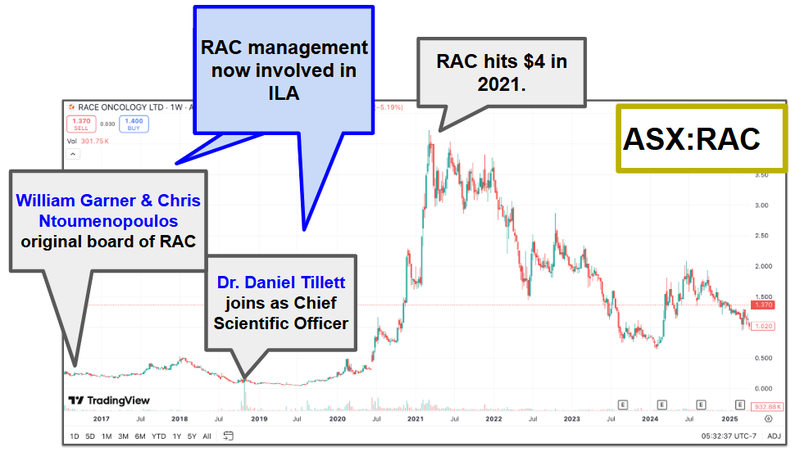
The past performance is not and should not be taken as an indication of future performance. Caution should be exercised in assessing past performance. This product, like all other financial products, is subject to market forces and unpredictable events that may adversely affect future performance.
Here are the ILA substantial shareholders and board members:

(Source)
What’s the size of the prize?
There are two ways in which ILA develops value from this product.
1. Priority Review Voucher
A priority review voucher is a “reward” for biotech companies that develop drugs that the FDA are targeting... in particular:
- Neglected tropical diseases
- Rare pediatric diseases
- Medical countermeasures (e.g. for bioterrorism or pandemics)
FDA Priority Review Vouchers can either be used for the specific drug that the company is seeking to be approved, OR be used on the next drug that the company is looking to develop (like a bonus ‘thank you’ for getting approved).
The priority review reduces the review time frame from 10 months down to 6 months.
The reason PRVs are so valuable is because:
- A drug can get put into market a lot quicker than the traditional commercialisation strategy. Time is money.
- Once a company is granted PRV status for a drug, that status can be transferred to another product of choice without having to qualify for a priority review.
That time saving is obviously valuable for companies who can get into market ~4 months earlier (versus going through the priority review process).
Particularly to big pharma, where new drugs can generate billions of dollars in revenue a year and there is a 7-year exclusivity timeframe before generics hit the market.
PRVs have sold for as high as US$350M (source).
ILA is hoping to fast track approvals and secure a PRV for Galidesivir because of:
- The existing safety and pharmacogenetic data conducted in two Phase 1 clinical trials by BioCryst (the company ILA bought the drug rights from),
- The “Animal Rule” which allows effective drugs to be approved just from animal trials.
These two factors may significantly shorten the timeframe for ILA to secure FDA approval and the coveted PRV.
A couple of weeks ago, the FDA Commissioner highlighted the potential for new Priority Vouchers to be granted to “companies supporting the US National Interest”.

(Source)
There were four broad categories that were identified by the FDA as “US National Interest”:
- Addressing a health crisis in the U.S.
- Delivering more innovative cures for the American people.
- Addressing unmet public health needs.
- Increasing domestic drug manufacturing as a national security issue.
While these categories don’t necessarily give a specific direction to the market as to which types of drugs will be eligible, the prize is big.
However, the possibility that ILA is eligible for two different Priority Vouchers is a big prize worth chasing.
Here is a recent sale of PRV for US$160M.
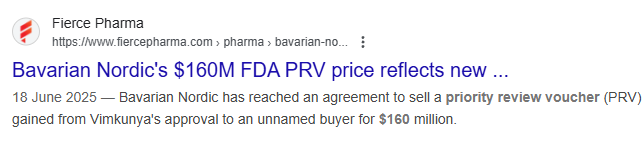
(Source)
2. Government Stockpiling
The big upside is that Galidesivir could make ILA a “stockpiling play”.
This is where the drug is of such interest and importance to public health that governments have a strong interest in stockpiling a reserve “in case of emergency”.
The best way to think about it is if we could have foreseen a big COVID outbreak in 2020 and the vaccine already existed, governments could have used their stockpile to prevent the spread of the outbreak.
This would have avoided all of the disruptions caused by the lockdowns and vaccine rollout.
OR even worse, in the event a bioweapon is used in war...
Governments wouldn’t be scrambling for a defensive vaccine/treatment if they had stockpiles.
Here are some government stockpiling deals that have been awarded in the last few years:
- Emergent BioSolutions up to $911 million through 2021 for BioThrax (the only FDA anthrax vaccine). BARDA also awarded Emergent $100 million for BioThrax.
- Bavarian Nordic- $120million for JYNNEOS (smallpox and mpox vaccine) in 2023
- Bavarian Nordic- $140million for JYNNEOS in 2024; BARDA also awarded them $63 million
- Bavarian Nordic- $140million for JYNNEOS in 2025
Here is a slide from ILA’s recent presentation which shows stockpiling sales for other drugs:
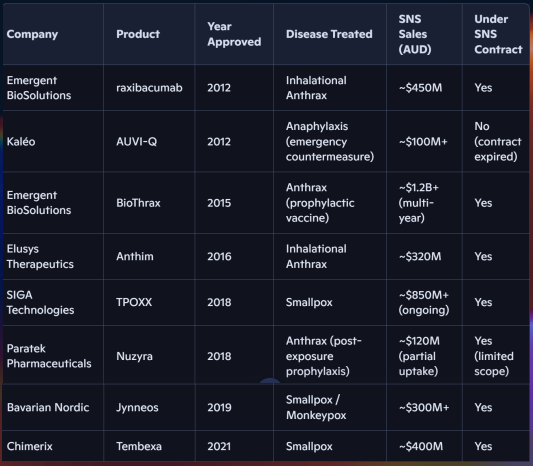
(Source)
What’s next for ILA in the next 6-9 months
Today: ILA confirms Animal Rule eligibility for its Marburg drug ✅
NEXT: FDA confirms to ILA the use of the Animal Rule for its Marbourg disease treatment and PRV voucher eligibility successful.
NEXT: Commence animal trial for deadly Marburg disease. Here are the milestones we will be tracking for the animal study:
- 🔲 Clinical trial design completed
- 🔲 Clinical trial starts
- 🔲 Clinical trial completed
- 🔲 Clinical trial results
NEXT: (Assuming results are good) Submit to FDA for commercial approval and access to Priority Review Voucher
What are the risks?
The primary risk for ILA is regulatory uncertainty.
While the FDA’s Animal Rule provides a potential fast-track pathway for Galidesivir, it is not guaranteed.
ILA must meet with the FDA to determine if the existing non-human primate efficacy data and Phase 1 human safety data from the previous owner are sufficient to proceed.
If not, the company may be required to conduct new studies, increasing both costs and timelines.
The second risk for ILA is efficacy risk, although there is some good early stage data, ILA will need to prove in a larger clinical trial that Galidesivir is effective.
The third risk is commercialisation risk, even with approval, there is no certainty that ILA will receive a Priority Review Voucher or secure government stockpiling contracts.
The fourth risk is competition risk, there are other drugs being developed for Marburg, and if they make it to market first, it may affect the commercial potential of Galidesivir.
Other risks:
Like any stock market investment, investing in ILA carries a multitude of risks which may affect the value of the company, some of which cannot be foreseen (this is the nature of risks).
Here we aim to identify a few more risks.
ILA is a small-cap biotech company, meaning it is highly speculative and subject to sharp swings in sentiment around trial results, FDA feedback, or broader biotech market conditions.
The company is reliant on timely execution of its clinical and regulatory milestones. Any delay in feedback from the FDA, setbacks in the planned animal trial, or additional data requests could materially impact the investment case.
ILA will require continued access to funding as it advances its programs. While currently funded for its near-term studies, any unexpected trial or regulatory costs may force ILA to raise additional capital, which could dilute existing shareholders.
ILA is also exposed to geopolitical and government funding risks. Stockpiling contracts are ultimately dependent on US government priorities and budget allocations, which may shift over time.
Finally, the current share price may already be factoring in the possibility of a Priority Review Voucher or lucrative stockpiling contracts. If either of these does not eventuate, the downside could be significant.
Investors should carefully consider these risks and seek professional advice tailored to their circumstances before investing.
Our ILA Investment Memo
You can read our ILA Investment Memo here. We use this memo to track the progress of all our Investments over time.
Our ILA Investment Memo covers:
- What does ILA do?
- The macro theme for ILA
- Our ILA Big Bet
- What we want to see ILA achieve
- Why we are Invested in ILA
- The key risks to our Investment Thesis
- Our Investment Plan
General Information Only
This material has been prepared by StocksDigital. StocksDigital is an authorised representative (CAR 000433913) of 62 Consulting Pty Limited (ABN 88 664 809 303) (AFSL 548573).
This material is general advice only and is not an offer for the purchase or sale of any financial product or service. The material is not intended to provide you with personal financial or tax advice and does not take into account your personal objectives, financial situation or needs. Although we believe that the material is correct, no warranty of accuracy, reliability or completeness is given, except for liability under statute which cannot be excluded. Please note that past performance may not be indicative of future performance and that no guarantee of performance, the return of capital or a particular rate of return is given by 62C, StocksDigital, any of their related body corporates or any other person. To the maximum extent possible, 62C, StocksDigital, their related body corporates or any other person do not accept any liability for any statement in this material.
Conflicts of Interest Notice
S3 and its associated entities may hold investments in companies featured in its articles, including through being paid in the securities of the companies we provide commentary on. We disclose the securities held in relation to a particular company that we provide commentary on. Refer to our Disclosure Policy for information on our self-imposed trading blackouts, hold conditions and de-risking (sell conditions) which seek to mitigate against any potential conflicts of interest.
Publication Notice and Disclaimer
The information contained in this article is current as at the publication date. At the time of publishing, the information contained in this article is based on sources which are available in the public domain that we consider to be reliable, and our own analysis of those sources. The views of the author may not reflect the views of the AFSL holder. Any decision by you to purchase securities in the companies featured in this article should be done so after you have sought your own independent professional advice regarding this information and made your own inquiries as to the validity of any information in this article.
Any forward-looking statements contained in this article are not guarantees or predictions of future performance, and involve known and unknown risks, uncertainties and other factors, many of which are beyond our control, and which may cause actual results or performance of companies featured to differ materially from those expressed in the statements contained in this article. S3 cannot and does not give any assurance that the results or performance expressed or implied by any forward-looking statements contained in this article will actually occur and readers are cautioned not to put undue reliance on forward-looking statements.
This article may include references to our past investing performance. Past performance is not a reliable indicator of our future investing performance.

